Tuberculosis
Challenges include under-diagnosis, as many people are unaware that they have the disease, and the emergence of treatment-resistant forms.
Only around two out of five people with drug-resistant tuberculosis had access to treatment in 2022. In recent years, major progress has been made in the fight against tuberculosis, in particular by offering shorter and more effective treatments for resistant forms, but questions remain about their use in the most vulnerable populations.
Tuberculosis (TB) is caused by Mycobacterium tuberculosis, a bacteria most commonly affecting the lungs. Although tuberculosis is largely preventable and curable, by 2015, 10.6 million people contracted the disease in 2022 and 1.3 million died from it (WHO Global Tuberculosis Report). Almost one quarter of the world's population has latent tuberculosis infection and could develop the disease. In sub-Saharan Africa, the burden of TB is mostly driven by the HIV epidemic.
It’s been said that tuberculosis is a disease of poverty, as 95% of TB deaths occur in low- and middle-income countries. In particular children, HIV-infected persons, people suffering from malnutrition and prisoners, are at risk of TB. This may be due to lack of access to adequate diagnosis and treatment or to poor response to treatment. In addition, the emergence of resistance to two major anti-tuberculosis drugs, isoniazid and rifampicin (Multidrug resistant tuberculosis or MDRTB), is posing a major problem in tuberculosis control.
Are the latest innovations effective in vulnerable populations?
In the last few years there has been progresses in the diagnosis and treatment of TB. However, many of these new tests and treatment regimens have not been evaluated on the populations that could benefit the most. Epicentre works with Médecins Sans Frontières (MSF) measuring the importance of the disease in vulnerable populations, evaluating the implementation of new drugs, novel regimens, new diagnosis tests, and novel case management strategies in order to improve the patients’ outcomes.
Diagnosis remains a challenge
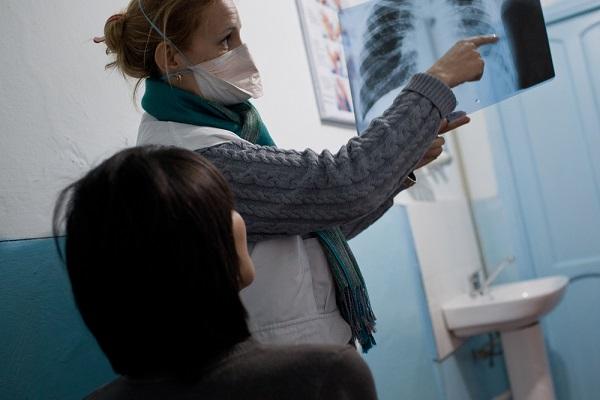
TB diagnosis remains a challenge in resource constrained settings, especially in children and advanced HIV-infected patients.
Epicentre has conducted several observational studies on the accuracy, the diagnostic value, the cost-effectiveness and the feasibility of using the point-of care urine based Determine TB-LAM (commercialized by Alere/Abbott), in HIV-infected patients in Kenya, Mozambique, Malawi and DRC. The same test has also been evaluated in severely malnourished children in Niger and in severely ill children in Uganda. The test has been found to have modest sensitivity but important diagnostic value for TB in HIV-positive patients. It is also feasible to implement in low resources settings and highly cost-effective.
Epicentre also conducted a study to evaluate a new point-of-care urine-based TB-LAM test (developed by Fujifilm and not commercialized yet), the FujiLAM, in HIV-positive adults. Funded by the French National Agency for AIDS Research (ANRS) and viral hepatitis and MSF, the study took place in four sites with high HIV and TB prevalence: Uganda, Kenya, Mozambique and South Africa. Results indicate a high FujiLAM testsensitivity in HIV-positive TB patients and higher than that observed with, the currently recommended test Determine TB-LAM (Alere/Abott). The FujiLAM test is also feasible to implement, well accepted by test users and by patients.
Therapeutic decision algorithms: hope for the diagnosis of tuberculosis in children.
To date, there are no simple, reliable, inexpensive, and easily accessible diagnostic tests to detect tuberculosis in children. The diagnostic tools available are insufficient, and rarely integrated into primary health care, where most children with tuberculosis seek treatment. As a result, children are often diagnosed very late, and for those who are most at risk of developing the most severe forms of tuberculosis, these delays are often fatal. However, if children are diagnosed all anti-tuberculosis drugs approved for use in children are now available in pediatric formulations.
This situation could undergo a major change thanks to the incorporation of new recommendations from the World Health Organisation (WHO), including new treatment decision algorithms and shorter therapeutic strategies. One of the great strengths of these algorithms lies in their ability to be deployed in contexts lacking specialized diagnostic tools, by non-specialized teams, and as close as possible to patients.
The TACTiC project - Test, Avoid, Cure TB in Children - launched at the end of 2023 by Médecins Sans Frontières (MSF), plans to implement recommendations in 12 countries in Africa and Asia. These algorithms, although relatively simple and not requiring complex laboratory analyses, remain largely under-utilized and have not yet been evaluated.
The TACTiC project therefore includes an operational research component led by Epicentre, comprising a multi-country diagnostic study to assess the performance of these algorithms and a mixed methods study to assess the performance of these algorithms. These studies are taking place in 5 countries with distinct paediatric care contexts:
- Maiduguri in Nigeria since August 2023,
- Madarounfa in Niger and Conakry in Guinea since September 2023,
- Malakal in South Sudan and Mbarara in Uganda since October 2023.
Simplifying diagnosis through imaging
Countries with a high prevalence of tuberculosis often have a shortage of X-ray machines, due to their cost and the need for staff trained to use them and interpret the images, which further hampers the diagnosis of tuberculosis. In Papua New Guinea, a prospective study compared the results obtained by an affordable portable ultrasound machine with those obtained by chest X-ray in the diagnosis of pulmonary tuberculosis in people aged 15 and over with suspected tuberculosis who were referred for diagnosis to the MSF clinic in the Gerehu General Hospital. In Manila in the Philippines, Epicentre is evaluating whether the implementation and use of chest radiography assisted by an artificial intelligence algorithm to interpret images is feasible and acceptable.
New therapeutic approaches
TB-speed: diagnosing to stop TB in children
The Mbarara center was heavily involved in the TB-Speed consortium led by IRD and the University of Bordeaux on the management of pediatric tuberculosis and the improvement of early detection. In particular, this study demonstrated the feasibility of detecting tuberculosis in children hospitalized for severe pneumonia, the good performance of a simplified stool sample preparation method for the molecular diagnosis of tuberculosis, the evaluation of diagnostic algorithms to guide rapid treatment decisions in vulnerable children living with HIV or suffering from severe acute malnutrition, and the feasibility of decentralizing care to the community level. In particular, the results of the TB-Speed study argue in favor of more systematic use of the Xpert Ultra rapid test in these children, especially those suffering from severe acute malnutrition.
Datura: more intense treatment in HIV-positive patients with tuberculosis
Funded by EDCTP and ANRS, the DATURA trial - Determination of Adequate Tuberculosis Regimen in Adults and adolescents hospitalized with HIV-associated severe immune suppression - aims to assess whether more intensive initial treatment of tuberculosis, consisting of increased doses of the main antibiotics used, rifampicin and isoniazid plus corticosteroids, increases the chances of survival of hospitalized adults and adolescents co-infected with HIV and tuberculosis, as compared to standard treatment of TB. The Mbarara Research Center is one of the study sites.
Promoting household TB contacts’ screening and preventive treatment coverage
The Mbarara centre also took part in the CONTACT study coordinated by IRD, which is part of the the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF)’s CaP-Tuberculosis (TB) Project and was funded by global health organization Unitaid.
This is the first cluster randomized controlled trial to evaluate a community-based intervention for tuberculosis screening and TPT management of household child contacts by community-health workers.
The study compared its effectiveness, feasibility, and cost-effectiveness to the facility-based standard of care. The study was performed in Cameroon and Uganda and shows that community-based contact investigations performed by community-health workers increases the coverage, initiation, and completion of preventive treatment of tuberculosis (TPT) among child contacts under 5 years old or 5-14 years old children living with HIV. The proportion of child contacts of all ages who were screened for TB increased from 47.3% in the standard of care arm to 81.9% in the intervention arm and the proportion of child contacts in the TPT target group that initiated and completed TPT increased from 61.7% in the standard of care arm to 79.9% in the intervention arm.
The intervention prevented 15 tuberculosis deaths in Cameroon and 10 in Uganda. The incremental cost-effectiveness ratio was $620 per disability-adjusted life-year (DALY) averted in Cameroon and $970/DALY averted in Uganda.
The CONTACT study confirms that decentralizing contact investigation at the household level using community-health workers is feasible and effective in improving child contact screening coverage, TPT uptake and completion as compared to facility-based standard of care.
Drug-resistant TB is on the rise
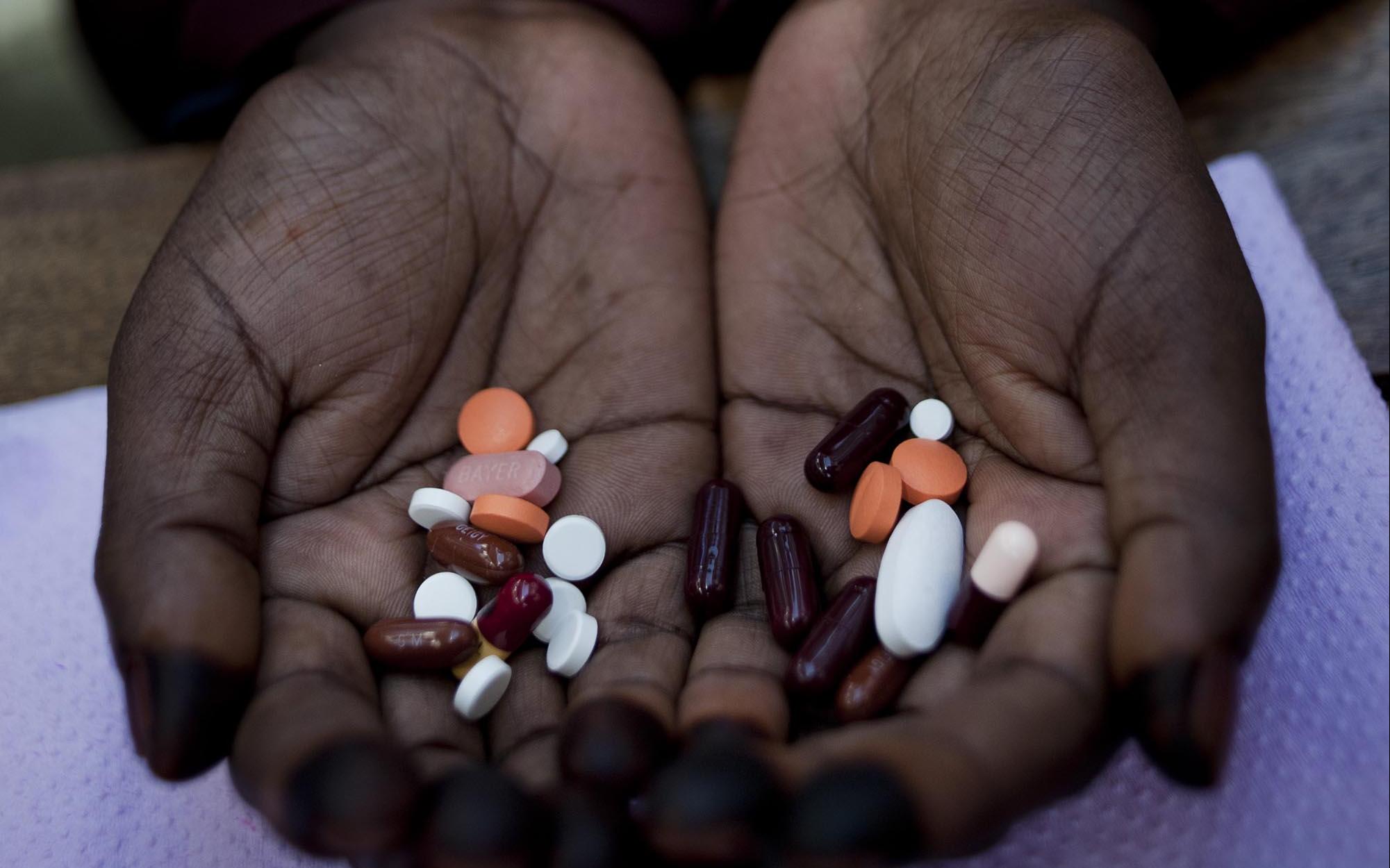
The emergence of resistance to anti-tuberculosis drugs is a global threat to the fight against tuberculosis. Resistance to one or more antibiotics is already observed and more worryingly, more and more multidrug-resistant strains, i.e., those that have become insensitive to several drugs, including at least the two most effective (isoniazid and rifampicin), are emerging.
In 2022, according to the WHO, 410,000 people developed multidrug-resistant tuberculosis or tuberculosis resistant to rifampicin, the most effective first-line drug.
Despite progress, treatment failure for people diagnosed with a resistant form of the disease remains high: worldwide, in 2020, the treatment success rate was 63%, compared with 60% in 2019 and 50% in 2012.
Until recently, treatments for multidrug-resistant tuberculosis can last up to 24 months. They are often accompanied by significant side effects. For some patients, they result in the ingestion of 14,000 pills and painful daily injections for months. The cost, difficulty and duration of these treatments make them difficult to implement.
Significant progress has been made in the last two years, thanks in particular to two studies piloted by MSF. The first showed that the BPaLM regimen, a six-month oral treatment combining bedaquiline and pretomanid with the older drugs linezolid and moxifloxacin, had an 89% cure rate in the TB-PRACTECAL trial. Recognising its many advantages - highly effective, shorter, injection-free, less toxic, with options for everyone - the World Health Organisation (WHO) now recommends BPaLM as first-line treatment for multidrug-resistant tuberculosis in people aged over 14.
EndTB: shorter, less toxic and more effective treatments for "multidrug-resistant tuberculosis" (MDR-TB).
the other study, EndTB, is a large project which took place in 17 countries and involved Partners In Health, Médecins Sans Frontières, Interactive Research & Development. Funded by UNITAID, it is organized around an observational study and 2 clinical trials sponsored by MSF and in which Epicentre, Harvard Medical School and the Institute of Tropical Medicine in Antwerp are involved.
The trial found three new drug regimens that can deliver similar efficacy and safety to conventional treatments while reducing treatment time by up to two-thirds. Regimens 1, 2, and 3 achieved favorable outcomes in 89.0%, 90.4%, and 85.2% of participants, respectively.
The endTB regimens offer treatment options, including for children under 14 and pregnant women for whom pretomanid is not recommended. In August 2024, the results of the endTB trial led the WHO to declare, for the first time, that evidence encourages it to recommend shorter treatment regimens for new population groups such as children, adolescents, pregnant and breastfeeding women. These groups have traditionally been excluded from therapeutic innovations, or have had access to them only at a late stage.
In addition, the trial supports the use of a fourth regimen as an alternative for people who cannot tolerate bedaquiline or linezolid; at least one of these two drugs is in every current World Health Organization-recommended regimen for MDR-TB.
The observational study in which Helena Huerga, an epidemiologist at Epicentre, is one of the principal investigators aims to collect data on the safety and efficacy of protocols using the drugs authorized in 2012 and 2013, respectively, bedaquiline and delamanid, for multidrug-resistant tuberculosis. The interim results show these two molecules to be safer than the drugs currently in use and suggest an improved response to treatment of resistant forms of TB. However, these results are not homogeneous across countries.